 Liquid Fuel Combustion: Mixture
Modelling using Continuous
Thermodynamics
Liquid Fuel Combustion: Mixture
Modelling using Continuous
Thermodynamics Liquid Fuel Combustion: Mixture
Modelling using Continuous
Thermodynamics
Liquid Fuel Combustion: Mixture
Modelling using Continuous
Thermodynamics
Dr. W. Hallett
Most commercial liquid fuels are mixtures with large numbers of components, often hundreds if all isomers are counted. Despite this, there is little information on the evaporation, ignition and combustion of such mixtures, and realistic models of petroleum fuels for use in spray combustion codes are not available. Most work on multicomponent fuels has been restricted to binary mixtures, but these are inadequate representations of real fuels. There have been few studies of more complex systems, and there have been no really satisfactory methods of making calculations and simulations of real fuel behaviour in combustion systems. "Continuous thermodynamics" offers a powerful method of modelling complex mixtures such as commercial fuels. It treats the mixture composition as a probability density distribution rather than as a series of discrete components, with the distribution giving the composition as a function of a property such as species molecular weight or boiling point. This is a powerful technique for modelling commercial fuels, whose large number of components makes a detailed description impractical.

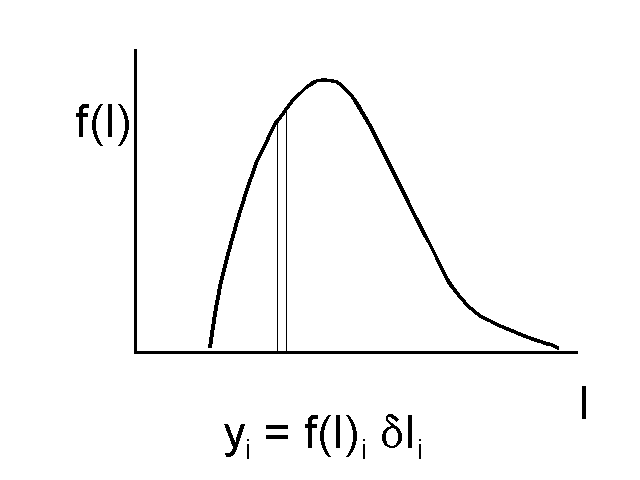
The sketches show the basic principle of continuous thermodynamics. The actual mixture (left) contains a large number of hydrocarbon components (eg. heptane, octane, nonane, etc), far more than one can conveniently measure or make calculations for. They are shown here as concentrations plotted versus a distribution variable I, which could be component molecular weight, carbon number, or boiling point. These concentrations are represented in continuous thermodynamics with a continuous function (right), so that the concentration of a component with a particular value of I is now given by a thin slice out of the PDF. Now, instead of requiring the concentrations of dozens of components. one only needs the parameters of the distribution function (mean, standard deviation) to make calculations for the fuel.
In the last three decades, continuous
thermodynamics has found widespread use for chemical
process design in petroleum refining, for describing natural gas
liquids and crude oils, and for
dealing with polymer reactions. Prior to our work, it had not been
applied to combustion
problems. We began by deriving a series of transport equations which
are necessary to describe
the fuel vapour composition around the droplet. Essentially, these
equations allow the
distribution function to diffuse rather than the individual fuel
components. This series of
equations gave us a complete model for the evaporation of a droplet. We
were subsequently able
to simplify this so that it can be more easily used for practical
calculations. Our work has been used by a number of other research
groups in the US and in Europe.
Our current work has focussed largely on alternative fuels, mostly of biological origin: biomass pyrolysis oil, biodiesel, and ethanol/petroleum mixtures. Pyrolysis oil is a particularly complex mixture, containing many different chemical groups and a pyrolytic lignin component which pyrolyzes during droplet evaporation to produce a solid carbon residue. We have succeed in modelling these processes using continuous thermodynamics. We have also been looking at models for mixing in the liquid phase. We are also working on continuous thermodynamics representations of chemical kinetics, primarily through modelling of droplet ignition.
Return to Dr. Hallett's home page
Last Update: August 2010