 CHG 4127: Dr. D.G. Taylor
CHG 4127: Dr. D.G. Taylor CHG 4127: Dr. D.G. Taylor
CHG 4127: Dr. D.G. Taylor
Assignment 1: Stoichiometry and Stoichiometric Tables
Due Time and Date
: Monday, Sept. 28th, 3:30 PM
![]()
Suppose that this reaction takes place at 8.2 atm and 227 oC using a feed consisting of 15 mol% ammonia in air. Consider individually a constant pressure batch reactor, a constant volume batch reactor, and a constant pressure flow reactor. In each case set up a stoichiometric table and express the partial pressure and concentration of each species (inert and reacting) in terms of the fractional conversion and other relevant quantities. Calculate these for fractional conversions of 0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, and 1 and plot your results.
![]()
is carried out at 200 oC and 2500 kPa in a flow reactor. The vapour pressure of the 1,2-dibromoethane at 200 oC is 506.5 kPa. Set up stoichiometric tables for pre- and post- condensation. What is the conversion of ethane when condensation first begins? Plot the concentration and molar flow rate of each species as a function of total conversion, assuming a stoichiometric feed that is free of inerts. Assume as well that the volumetric flow rate of the feed is 0.5 litres/s.
![]()
takes place in a cylindrical batch reactor that has one end fitted with a frictionless piston attached to a spring (see the figure below). As a result, the volume of the reactor is related to its pressure as follows:
![]()
where V is in ft3 and P is in atm. The reactor temperature is constant at 140 oF and its initial volume is 0.15 ft3. Further, equal amounts of species A and B are introduced to it at the start of operation. Finally, the reaction rate expression is given by
![]()
where
![]()
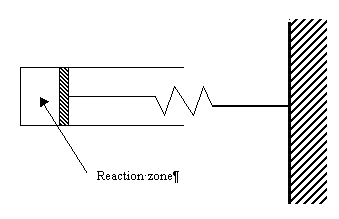
Schematic diagram of piston reactor for problem 3.