CHG 3316
TRANSPORT PHENOMENA
Assignment
#6
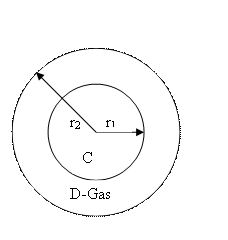
10-2. A droplet
of material C (radius r1) is suspended in
a gas stream of D which forms a stagnant film around the droplet (radius r2). If the C concentrations
are xc1 and xc2 at r1 and r2, find the flux of C.
Droplet At r = r1,
x = xc1
r = r2, x = xc2![]()
SOLUTION
Let Dcd be the diffusion coefficient.
ASSUMPTIONS
- One dimensional Diffusion (in the r-direction only)
- Steady state
- No reaction nor generation is taking place.
Based on the assumptions, equation 10-9
(Griskey) for cylindrical co-ordinates, becomes:
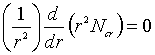
![]() (1)
(1)
![]()
![]() =
= ![]() /
/![]() .(2)
.(2)
From equation (10-6, Griskey) we have the definition of the flux:
![]()
But ![]() =0 (Stagnant film)
=0 (Stagnant film)
![]()
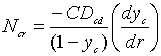 ..(3)
..(3)
Substituting (1) in (3), we will obtain:
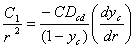
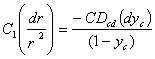 .(4)
.(4)
BOUNDARY
CONDITIONS
At r = r1, yc = xc1
r = r2, yc = xc2
, and by the Integration of Eq(4)
above:
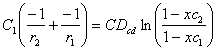
(![]() ) =
) = ![]()
(![]() ) =
) = ![]() and
and 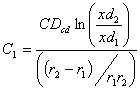
From (2), we have
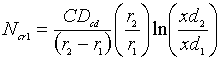 (Solution
for the flux)
(Solution
for the flux)
10-4. An
industrial pipeline containing ammonia gas is vented to the atmosphere (a 3 mm
tube is inserted into the pipe and extends for 20 m into air). If the system is
at 25oC, find the mass rate of ammonia lost from the vent.
SOLUTION
With the following data available:
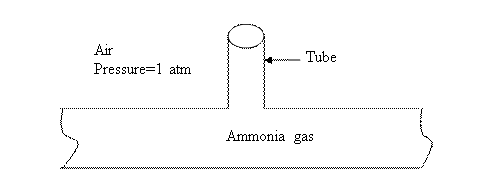
For the tube, Diameter, D=3 mm
Length, L=20 m
Temperature of system, T=25oC.
Diffusivity of Ammonia gas in tube,![]()
Molecular weight of Ammonia=17.03 g/mol
Molecular weight of Air=28.97 g/mol
Assume that Ammonia gas is diffusing in the y-direction only.
ASSUMPTIONS
- One dimensional diffusion
(y-direction)
- Steady state
- No reaction nor generation occurs
Based on the assumptions above, equation (10-7, Griskey) for rectangular co-ordinates becomes:
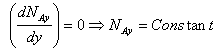 (1)
(1)
And the definition of flux, from equation (10-6, Griskey) for rectangular co-ordinates,
![]()
![]()
![]() =0 as there is no bulk flow.
=0 as there is no bulk flow.
![]() ..(2)
..(2)
![]()
Integrate the above equation ( EQ 2) with boundary conditions.
BOUNDARY
CONDITIONS
At L=0, CA=CAO
L=20 m, CA=
![]() ..(3)
..(3)
From ideal gas law, CA=P/RT
![]()
Substituting in (3),
![]()
We have![]()
![]()
Mass flow rate of Ammonia lost from Vent, ![]()
Where MA is the molecular weight of Ammonia
A is the area of the tube.
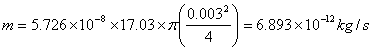
10-9
Benzene at 22°C is open to the atmosphere in a circular tank
(6.10 m in diameter). Vapor pressure and specific gravity for benzene are 0.132
atm and 0.88. An air film of 5-mm thickness is above the benzene. What is the
cost of evaporated benzene per day (assume value of benzene is $2 per gallon)?
![]()
Given Data:
Benzene Temperature = 22 C
Vapour pressure of Benzene = 0.132 atm
Specific gravity = 0.88
Air film of thickness = 5mm
Diameter of tank = 6.10 m
Cost of benzene = $ 2 per gallon
ASSUMPTIONS
· Diffusion of A molecules (Benzene) is only in one direction i.e. in the z direction no flux in x and y direction
·
Steady state with no chemical reaction i.e. ![]()
· The air film above can be assumed to be a stagnant layer of B molecules(non diffusing)
In this case, the system is a liquid A (benzene) evaporating into Gas B (air) i.e. diffusion through a stagnant gas film model.
Apply the above assumptions to the equation of continuity of species for rectangular coordinates (Equation 10-7 Griskey):
![]()
![]()
![]()
![]()
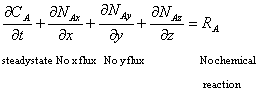
To obtain:
![]()
Therefore ![]() is independent of z
i.e. it is a constant
is independent of z
i.e. it is a constant
Then, using the Ficks definition of flux NA (Equation 10-6 Griskey):
![]()
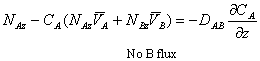
The equation above can be rearranged to get:
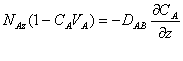
We can substitute the concentration term on the left side of the above equation with the mole fraction YA to get:
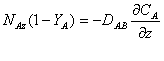 (1)
(1)
knowing
![]()
Replace the above equation into equation 1, we can derive the molar flux of A molecule in term of mole fraction YA:
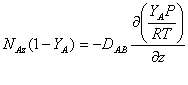
Since P, R and T are constant, they can be factored out of the derivative to obtain:
 ,,,,,,,
(2)
,,,,,,,
(2)
BOUNDARY
CONDITIONS
z =z1 YA=
YA1
z =z2 YA=
YA2
The equation obtained above (equation 2) can be integrated as follows (since NAz is constant):
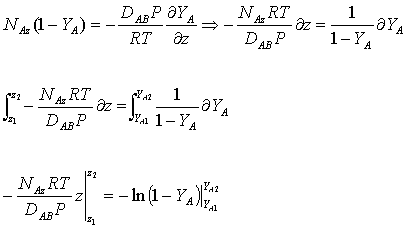
![]() ,,,,,,,,(3)
,,,,,,,,(3)
Letting ![]() (thickness) and
rearranging the equation above we obtain:
(thickness) and
rearranging the equation above we obtain:
![]() ,,,,,,,,,,,,,(4)
,,,,,,,,,,,,,(4)
The above equation of flux (Equation 4) can be written in terms of mole fraction for the stationary molecule i.e. YB as follows:
![]() ,,,,,,,(5)
,,,,,,,(5)
At the surface of benzene liquid:
The vapour pressure of benzene = 0.132 atm therefore:
![]() =0.132 and
=0.132 and ![]()
Assuming all the benzene is swept away by moving molecules of air above the stagnant air film i.e. the concentration of benzene above the air film is negligible, then:
![]()
Since the diffusivity of benzene is not given, it can be calculated from the following equation:
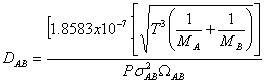
From A-3-3 in
Griskey:
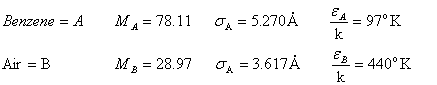
And,
![]()
![]()
To obtain![]() , table A-3-4 can be used using:
, table A-3-4 can be used using:
![]()
Therefore the diffusivity is:
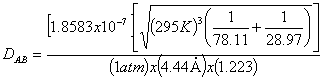
![]()
Now that we have all the parameters needed to determine the molar flux of benzene, we can substitute the above parameters into the molar flux equation derived earlier:
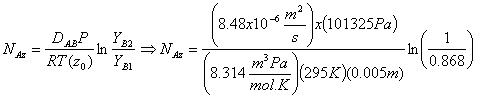
![]()
From the molar flux, the amount of benzene in gmols/sec can be determined as follows:
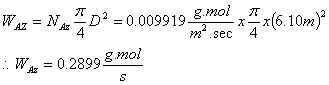
The amount of benzene evaporated
per day:
=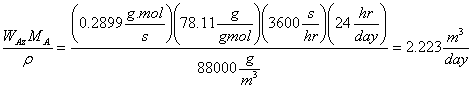
Gallons of benzene evaporated in one day:
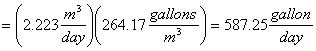
Therefore the Cost of benzene evaporated per day:
Cost of benzene evaporated = ![]()