Answers for Assignment 7
Problem 10-20: An alcohol and water vapor mixture is being separated by contact with an
alcohol-water liquid solution. Alcohol is transferred from gas to liquid, and
water is transferred from liquid to gas. Temperature and pressure are 25°C and
1 atm. The components diffuse through a gas film 0.1 mm thick at equal flow
rates. Mole percents of alcohol are 80 and 10 on either side of the film. Find
the rate of diffusion of both components through a film area of 10 m2.
Consider this to be a case of equimolar counter diffusion we did in the class:
We start from the modified equation 10-6:
![]()
Since we have
equimolar counter diffusion: NA
= - NB
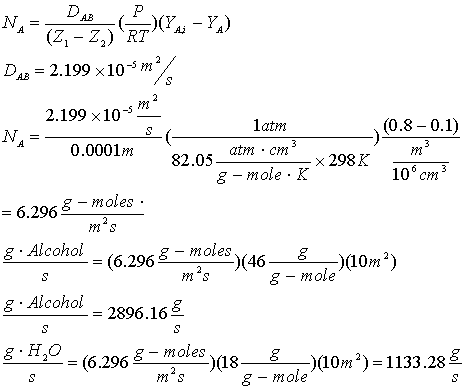
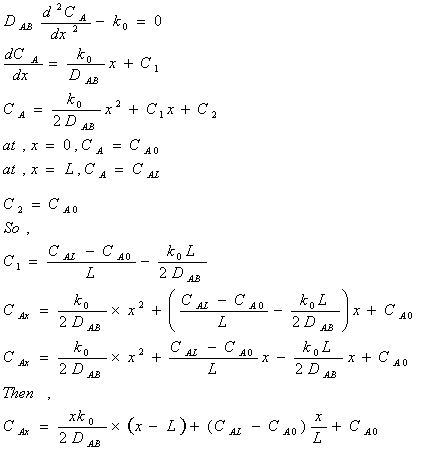
Problem 10-39: Oxygen is transferred from the inside of the
lung through the lung tissue to blood vessels. Assume the lung tissue to be a
plane wall of thickness L and that inhalation maintains a constant oxygen molar concentration at
the inner wall as well as another constant oxygen molar concentration at the
outer wall. Additionally, oxygen is consumed in the lung tissue by a metabolic
reaction (zeroth order). Determine the distribution of oxygen in the tissue and
the rate of assimilation of oxygen by the blood.
Solution:
In this case there is no bulk flow to be considered since we are dealing with membrane and negligible concentration of Oxygen within the membrane (i.e.: YA is very small in modified Equation 10-6. Also we have zeroth order reaction rate (RA=k0). Thus, at steady state, equation 10.7 simplifies to:
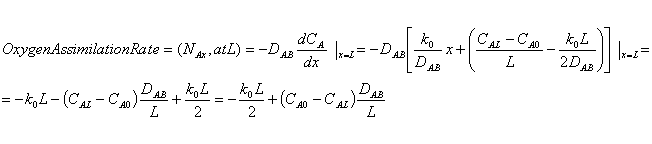
Problem 10-40: Carbonization of steel is a
high-temperature process. At a temperature of 1273 ºK, how much time would be
required to raise the steel carbon content at a depth of 1 mm from 0.1 to 1.0
percent (carbon mole fraction on steel surface is 0.02)? Take DAB =
3.17 x 10-11 m2/s.
y = 1 mm = 0.001m
![]() 3.17 x 10-11 m2/s
3.17 x 10-11 m2/s
x0 =
0.02
x1 =
0.001
x2 =
0.01
To use Figure 4.1-2 use C/C0 instead of vx/V and DAB instead of υ.
![]()
Then ![]() and
and ![]()
Since 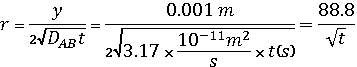
From the Unsteady –State Figure 4.1-2
given in the class for
rectangular Momentum Transfer for n=1:
![]() à r1
= 1.35 and
à r1
= 1.35 and ![]() = 0.5 à r2 = 0.48
= 0.5 à r2 = 0.48
![]()
![]() and
and
![]()
Total time = 34225 – 4327 = 29898 sec = 8.3 hours
Problem 4: Hydrogen gas (27°C; 10 bars) is stored in a 100-mm-diameter
spherical tank (2mm-thick wall). Molar concentrations of hydrogen at the inner
and outer wall are 1.5 kg-mole/m3 and 0,
respectively. Diffusivity of hydrogen in steel is 0.3x10-12 m2/sec.
Find the rate of hydrogen loss through the wall as well as the rate of pressure
drop in the tank at steady state. Because of the high ratio of the diameter of
the sphere to the thickness of the steel wall, do the same calculations,
assuming rectangular geometry for the 2 mm. thick steel wall the hydrogen is
leaking through and calculate the % error for making this assumption.
For Spherical:
Starting from modified equation 10.6:
![]()
In this case there is no bulk diffusion to be considered since we are dealing with membrane and negligible concentration of hydrogen within the membrane. Thus, equation 10.6 simplifies to:
![]()
![]()
![]()
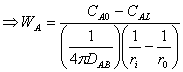
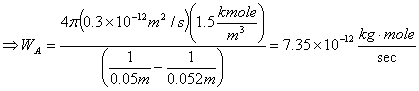
![]()
![]()
![]()
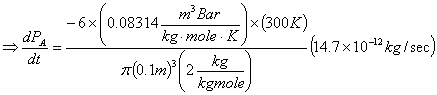
![]()
For
Rectangular:
Starting
from the modified equation 10.6 for binary system, again:
![]()
In this case there is no bulk flow to be considered since we are dealing with membrane and negligible concentration of hydrogen within the membrane. Thus, modified equation 10.6 simplifies to:
![]()
From equation 10-7 for a rectangular
system, NA=constant, so we can integrate the above equation:
![]()
![]()
![]()
Surface area of leak can be taken as the same as the sphere: 4πr2 = 4π(0.1/2)2m2 = 0.0314m2
![]()
![]()
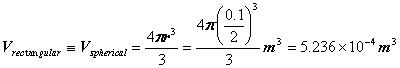
![]()
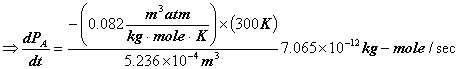
![]()
Assume 1 bar = 1 atm.
% error for hydrogen loss rate ![]()
% error for pressure drop rate ![]()