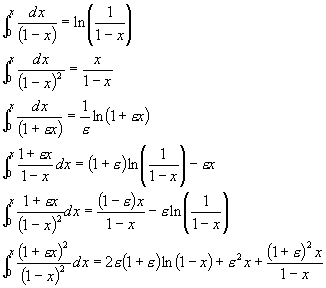Midterm Examination
Date
: November 2nd, 1998Permitted Aids
: TI30X calculator, Study Sheet (one sided)Answer all questions and show all work. Hand in study sheet with this exam.
Question 1
A catalytic, gas phase reaction
![]()
takes place in an isothermal plug flow reactor. The reactants enter as a stoichiometirc mix, together with an inert species at 55 oC and 5 atm. The mole fraction of the inert in the feed is 0.4
![]()
Neglecting pressure drop, how much catalyst is needed to convert 10% of the reactants to products?
Click here for the solution.
Question 2
The elementary reaction
![]()
takes place in the gas phase of a square duct. The feed to the duct consists of a gas stream of pure A and a liquid stream of pure B. The flowing liquid B covers the bottom of the duct and evaporates into the gas phase, maintaining its equilibrium pressure throughout the system. The gas phase, meanwhile, occurs as a plug flow. The volume of the liquid B is negligible relative to the gas phase.
Additional Data
P0 1 atm
k 106 ft3/lbmol•s
T 540 oF
Pv,B 0.25 atm at 540 oF
FA0 1.5 lbmol/s
Click here for the solution.
Other Useful Information for the Exam
R=0.082 (litre•atm)/(mol•K)=0.73 (ft3•atm)/(lbmol•oR)=8.309 (kPa•litre/mol•K)
o
R=oF+459.69 K=oC+273.16